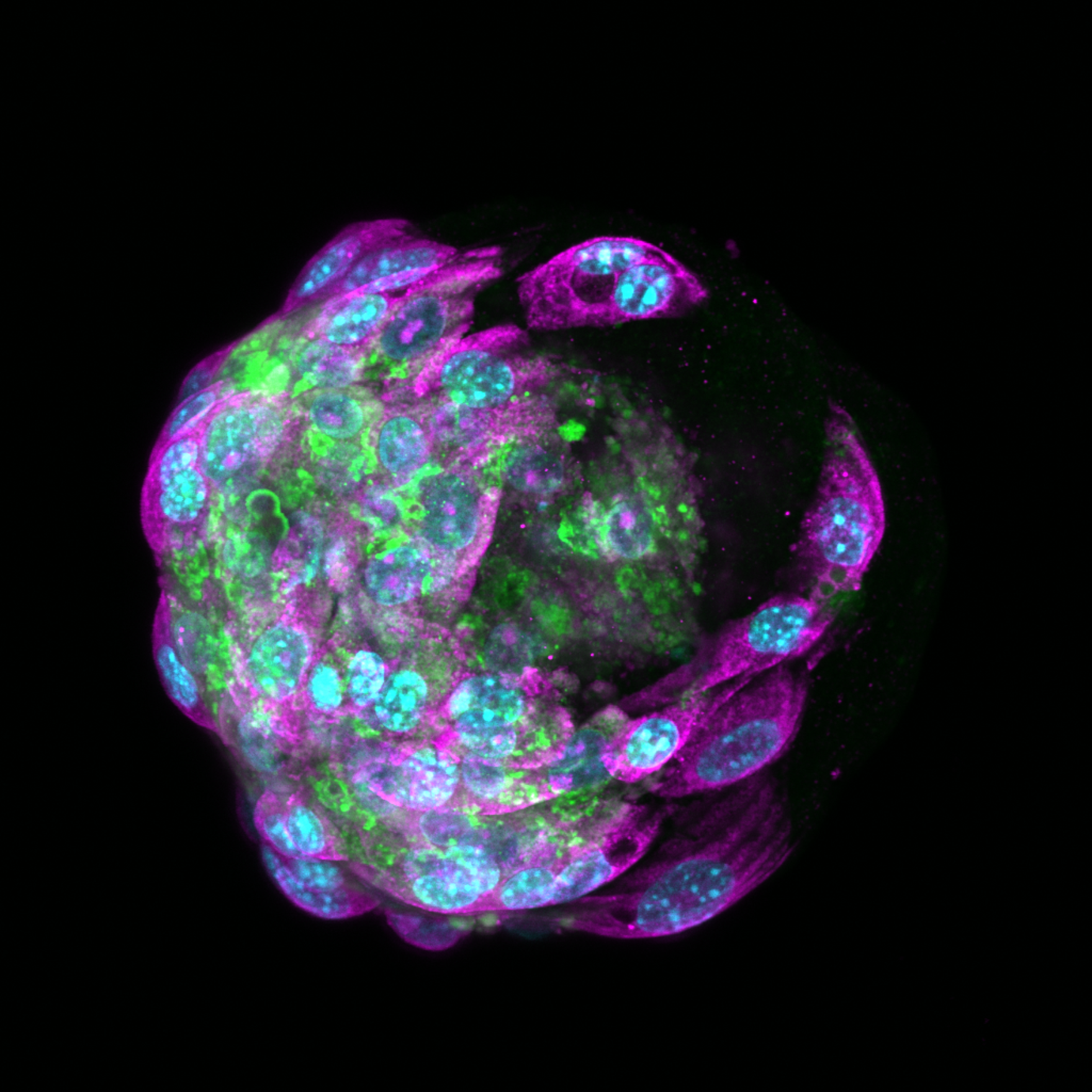
Recent advances in biofabrication are revolutionizing liver tissue engineering by enabling precise spatial patterning of liver cells to mimic the organ’s complex architecture. Techniques like 3D bioprinting, microfluidics, and self-assembled cell aggregates help recreate critical features such as metabolic zones, cell polarity, and vascular networks. These engineered liver models improve drug testing, disease research, and hold promise for regenerative therapies. Despite challenges in scaling and standardization, integrating multiple fabrication methods and emerging technologies like machine learning are driving progress. Ultimately, these innovations bring us closer to creating functional liver tissues for clinical and pharmaceutical applications. See full article here.
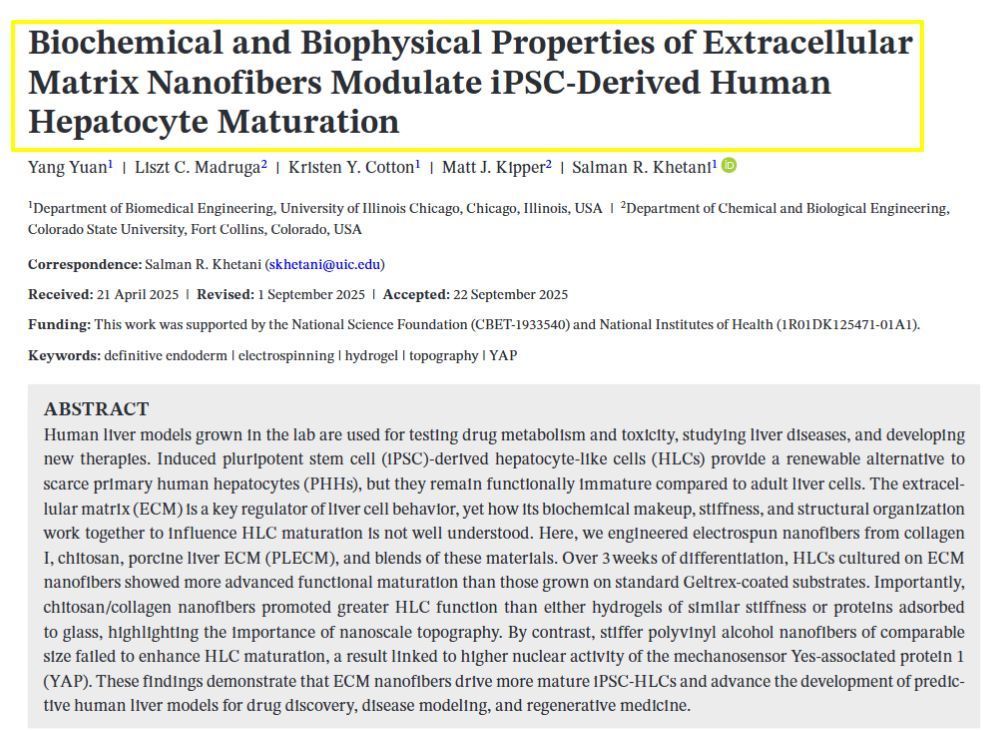
𝗦𝗶𝗺𝗽𝗹𝗲 𝗦𝘂𝗺𝗺𝗮𝗿𝘆: This study explores how we can improve lab-grown liver cells for medical research and drug testing. The MTMLab team works with induced pluripotent stem cells (iPSCs) - special cells that can be transformed into liver-like cells - because real human liver cells are hard to obtain. However, these lab-grown liver cells don't function as well as mature adult liver cells. The research team discovered that the surface environment where these cells grow is crucial for their development. We created tiny fiber scaffolds made from different materials like collagen, decellularized livers, and chitosan that mimic the natural structure around liver cells. When liver cells were grown on these specially designed nanofibers for three weeks, they displayed higher function compared to cells grown on standard surfaces. Our key finding was that both the material composition and the nanoscale fiber structure were important - stiffer synthetic fibers or softer materials without the appropriate topography or composition prevented proper cell maturation. This research helps create better lab models of human liver tissue that can be used for testing new drugs and studying liver diseases more effectively.

Owen Lally Modeling the synergistic effects of alcohol and fats on liver disease via engineered cocultures In Vitro Liver Toxicology Testing of Rat and Dog Hepatocytes to Reduce In Vivo Regulatory Requirements Nathan Shelton Enhancing the Functions and Hepatitis B Virus Infectability of Primary Human Hepatocytes Protein Microarrays to Probe Synergistic Effects of Extracellular Matrix Composition and Stiffness on Liver Macrophages Lesly Villarreal Engineering a 3D Placental Trophoblast Invasion Platform Via Droplet Microfluidics Gas-permeable Plates to Model Synergetic Effects of Oxygen and Endothelial Factors on Liver Zonation Emanuele Spanghero Modeling the Interplay Between Liver and Heart Diseases via a Human Dual-Organ Platform Engineering High Cell Density Beating Cords of Cardiomyocytes and Fibroblasts via Photopatterned Alginate
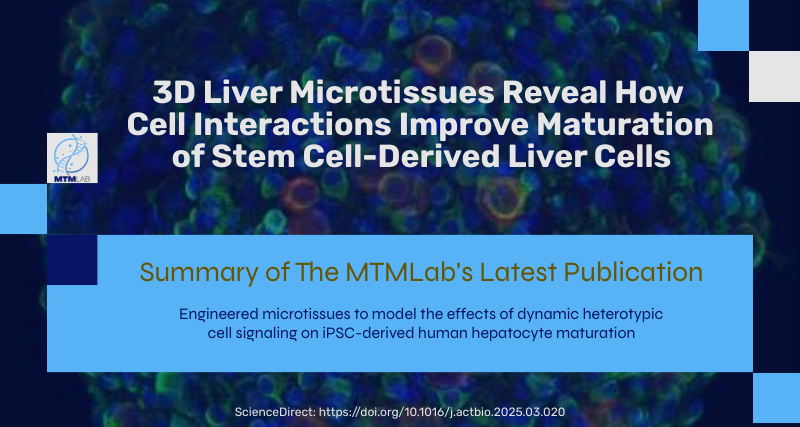
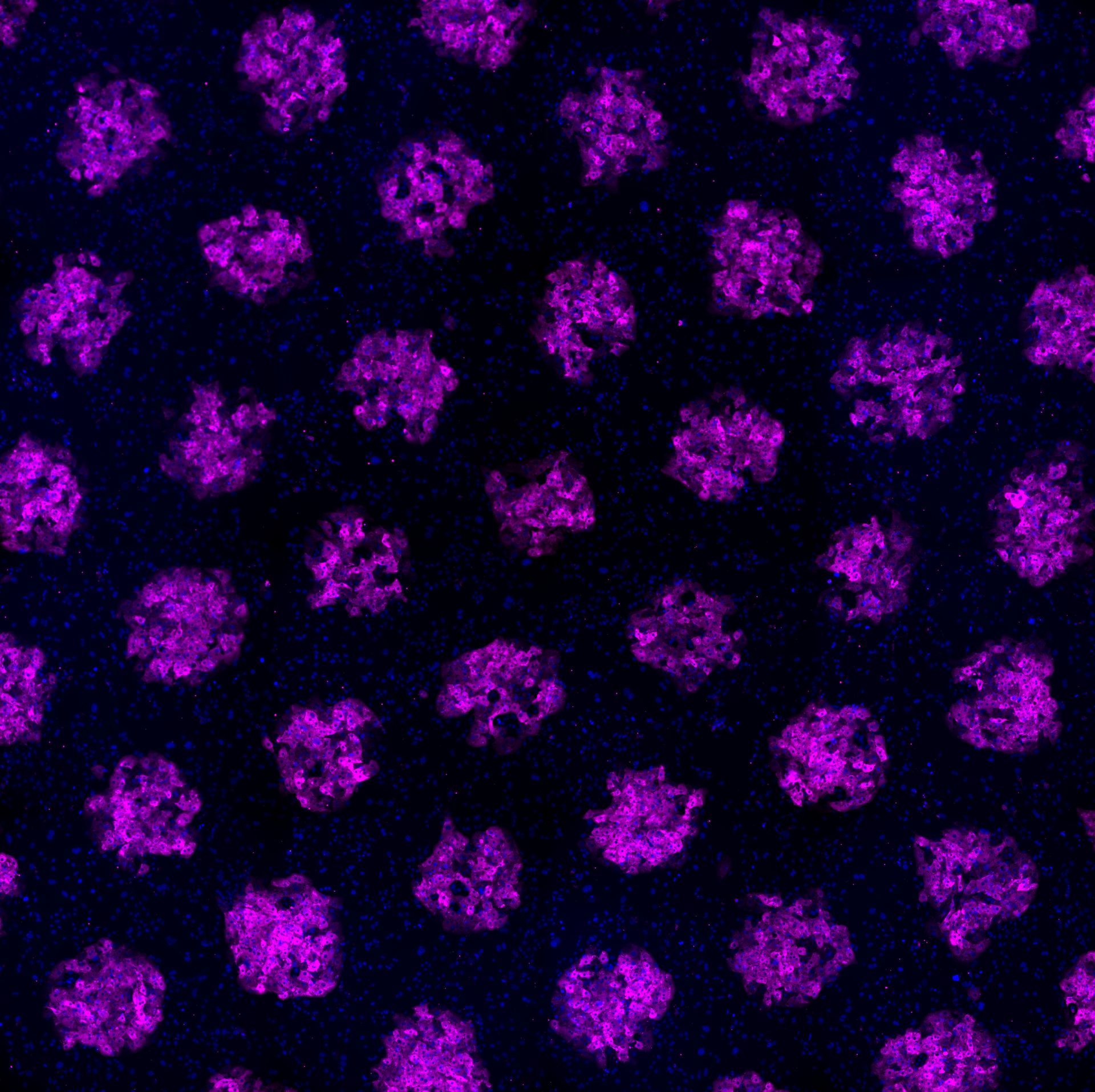
Our latest study addresses a critical challenge in liver tissue engineering: stem cell-derived liver cells (iHeps) typically remain functionally immature, limiting their usefulness for drug testing and disease modeling. Our research team created 3D microtissues using droplet microfluidics technology by: • Encapsulating iHeps in tiny collagen gel droplets (~250 μm diameter) • Coating these structures with various non-parenchymal cells (NPCs) • Testing different combinations and sequences of supporting cells Key findings: 1) Embryonic fibroblasts and liver sinusoidal endothelial cells (LSECs) produced the most mature iHeps compared to other tested cell types 2) Sequential application of cell signals (embryonic fibroblasts first, then LSECs) yielded optimal maturation 3) Specific growth factors like stromal-derived factor-1 alpha were identified as important maturation enhancers 4) Gene expression analysis confirmed that LSEC/iHep microtissues closely resembled adult human liver cells This platform enables researchers to identify critical cellular interactions and molecular signals that drive liver cell maturation, providing valuable insights for developing more physiologically relevant liver models for drug screening and regenerative medicine applications. https://www.sciencedirect.com/science/article/pii/S174270612500193X SIMPLE SUMMARY: Embryonic fibroblasts and liver sinusoidal endothelial cells dramatically improved iHep maturation compared to other cell types tested, producing more functionally mature liver cells. Sequential application proved crucial—adding embryonic fibroblasts first, followed by endothelial cells, yielded optimal maturation. Specific growth factors including stromal-derived factor-1 enhanced this process. This research enables creation of more authentic mini-liver tissues that function like human liver. These improved models support better drug testing, disease research, and regenerative medicine applications.

1. Three-Dimensional (3D) Cell Culture Techniques : New 3D cell culture methods have significantly improved the properties of stem cells, enhancing their viability and functionality for tissue regeneration. These techniques allow for more accurate modeling of tissue architecture and function. 2. Engineered Stem Cells : Advances in bioengineering have led to the development of "engineered stem cells," which are modified to enhance their regenerative capabilities. These next-generation stem cells are designed to be more effective in tissue repair and regeneration. 3. Injectable Biomimetic Hydrogels : Researchers have developed advanced injectable hydrogels that mimic natural tissue environments. These hydrogels hold significant promise for tissue engineering applications, providing a supportive matrix for stem cell growth and differentiation. 4. Integration with Tissue Scaffolds : There have been significant improvements in integrating stem cells with biomaterial scaffolds. These scaffolds provide structural support and enhance the differentiation and growth of stem cells into specific tissue types, improving the outcomes of regenerative treatments. 5. Gene Editing and mRNA Technologies : Techniques like CRISPR and mRNA-based therapies are being used to modify stem cells at the genetic level, enhancing their ability to regenerate tissues. These technologies allow for precise control over stem cell behavior and function.
2024 The MTM Lab has been awarded an NIDDK R01 (National Institute of Diabetes and Digestive and Kidney Diseases) grant to develop a novel microfluidic approach to elucidate the effects of soluble factor gradients, individually and in controlled combinations, on zonated functions in primary liver cells from rodents and humans towards determining species-specific effects . Ultimately, our novel devices can be used to investigate the mechanisms underlying liver zonation, chemical-induced zonated hepatotoxicity, and how zonation is perturbed in liver diseases, such as non-alcoholic fatty liver disease and hepatocellular carcinoma. The MTM Lab has been awarded a NIEHS (National Institute of Environmental Health Sciences) grant to develop a high throughput system to test placental cell invasion using a 3D placental microtissue coupled with hepatic liver biotransformation . This first-of-its-kind hepatic-placenta organ-tandem on a chip will simulate the liver metabolism that chemicals undergo in vivo prior to reaching the placental bed. This state-of-the-art in vitro platform will be the first step towards incorporating organism-level organization into reproductive risk assessment using a non-animal-based approach. The MTM Lab has been awarded a NIEHS (National Institute of Environmental Health Sciences) grant to develop a human gut-liver platform with microbiome interactions for in vitro toxicology . These first-of-its-kind scalable human gut-liver models will be developed for in vitro applications, such as compound screening and disease modeling, and be used to elucidate the effects of reciprocal tissue crosstalk on cell phenotype modulation. 2023 The MTM Lab has been awarded a NIDDK (National Institute of Diabetes and Digestive and Kidney Diseases) grant to analyze the synergistic effects of extracellular matrix composition and stiffness, multicellular interactions, and soluble triggers of NAFLD in cellular phenotypic alterations , which could aid the development of novel drug therapies for this disease. The MTM Lab has been awarded a NIAAA (National Institute on Alcohol Abuse and Alcoholism) grant to develop a first-of-its-kind organotypic mouse liver model and investigate the effects of alcohol on multiple liver cell types in this model with comparisons to an in vivo mouse model of ALD that recapitulates several key features of human ALD. This platform can aid in understanding the molecular mechanisms underlying alcohol-associated liver disease.
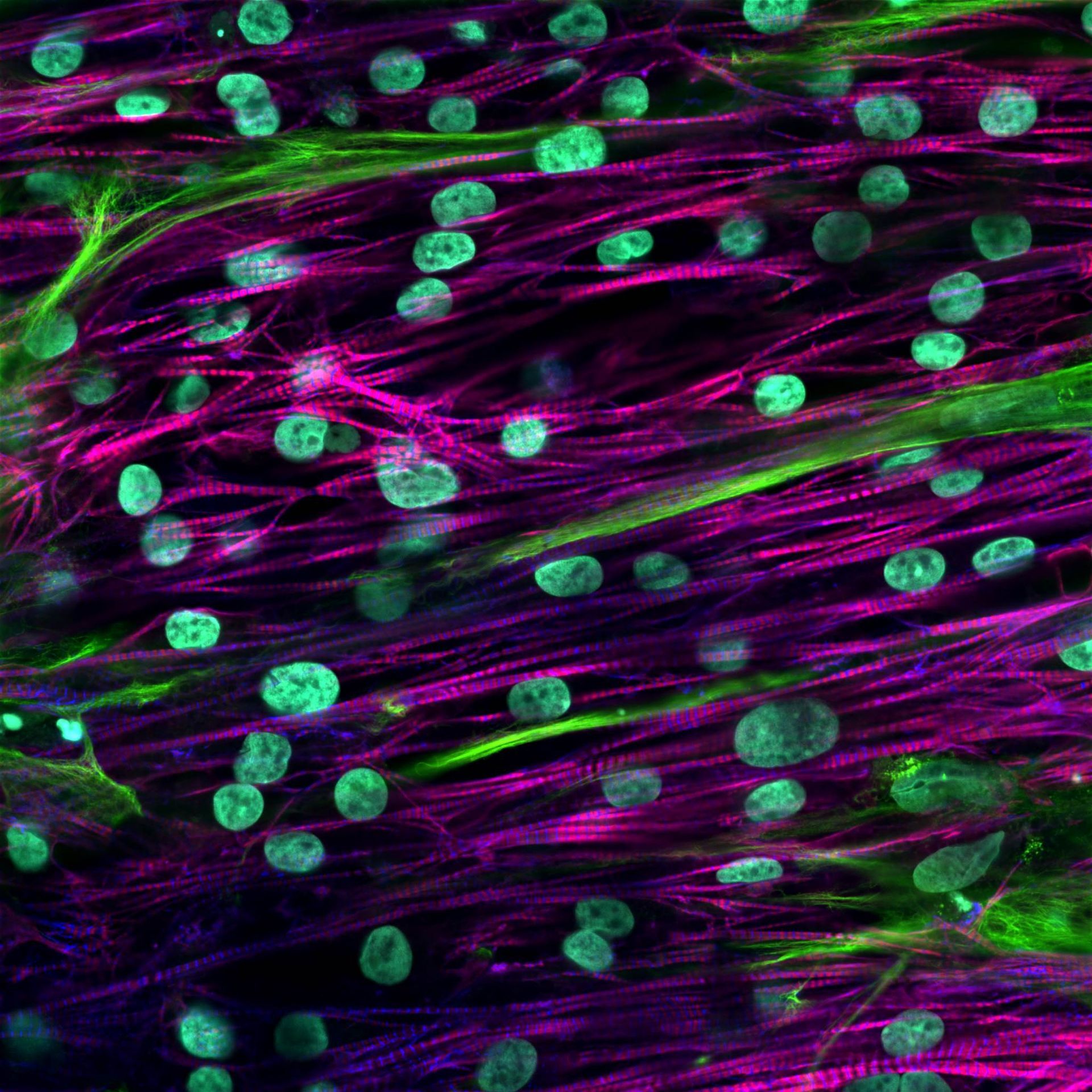
Students in the MTM lab presented 12 abstracts at the 2023 Annual Meeting of the Biomedical Engineering Society in Seattle, WA. Students in the MTM lab presented 11 abstracts in the form of 7 platform talks and 4 posters at the 2021 Annual Meeting of the Biomedical Engineering Society in Orlando, FL. Regeant Panday, a PhD student in the MTM lab, presented his work with 3D human liver tissues (poster) at the MicroTAS 2020 conference. The MTM lab presented 6 accepted abstracts (2 talks and 3 posters) at the Annual Meeting of the Biomedical Engineering Society. The MTM lab presented 8 abstracts, one as an oral presentation and seven as poster presentations at The Second Annual UIC Bioengineering Research Symposium. Congratulations to Grace Brown, Hardik Dabas, Demi Ibrahim, David Kukla, Jennifer Liu, Chase Monckton, Regeant Panday, and Yang Yuan for these presentations. The MTM lab presented 3 posters at the biannual meeting of the Center for Advanced Design and Manufacturing of Integrated Microfluidics (CADMIM) in Irvine, CA. Congratulations to Jennifer Liu, Grace Brown, and David Kukla for these presentations. The MTM lab presented 8 abstracts at the annual meeting of the Biomedical Engineering Society (BMES), incluidng 3 oral presentations and 5 poster presentations. Congratulations to Grace Brown, David Kukla, Jennifer Liu, and Chase Monckton for these presentations. Dr. Khetani presented MTM lab's research on a microfluidic human liver model at the annual meeting of the Biomedical Engineering Society (BMES) in Phoenix, AZ. David Kukla , Matt Davidson and Dr. Khetani presented MTM research in the form of oral talks and poster presentations at the annual meeting of the Biomedical Engineering Society (BMES) in Minneapolis, MN.
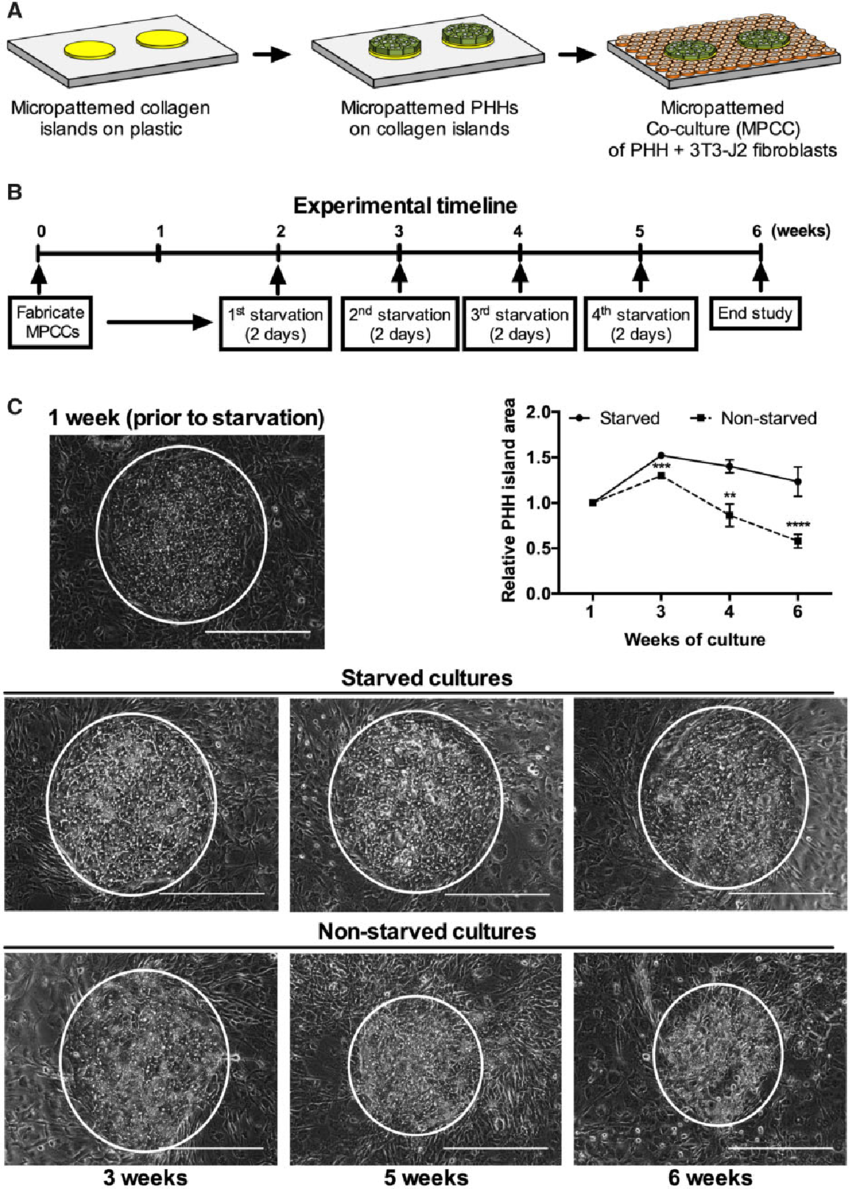
Primary human liver cells (PHHs) are crucial for testing harmful drug reactions in human patients. Normally, these cells lose their functions quickly in simple cultures, but when grown with other supportive cells, they last 2-4 weeks. However, since liver cells in the body function for much longer (200-400 days), we want to extend their functional life in the lab to study long-term drug effects and diseases. Fasting has been shown to improve the health & longevity of tissues like the liver. We thought that mimicking fasting in cell cultures might activate beneficial pathways and extend the life of PHHs. To do this, we periodically removed serum and hormones from a 'micropatterned coculture' of liver cells, which is a special arrangement of liver cells surrounded by connective tissue cells (fibroblasts) for support. A weekly 2-day starvation period extended the life of PHHs to over 6 weeks, compared to just 3 weeks without fasting. This fasting also improved the function of simpler 'monoculture' liver cells for 2 weeks. In the 'micropatterned cocultures', fasting activated a key energy-regulating protein called AMPK and prevented the supportive fibroblast cells from overgrowing, helping maintain liver cell structure (polarity). Similar benefits were seen when we activated AMPK with a drug called metformin or stopped the growth of supportive cells with another drug, mitomycin-C. Finally, liver cells in the fasting cultures were better at predicting drug toxicity and had higher drug-processing activities even after 5 weeks. In summary, intermittent fasting in cell cultures extends the functional life of liver cells, making them more useful for drug testing. Publication >> Intermittent Starvation Extends the Functional Lifetime of Primary Human Hepatocyte Cultures
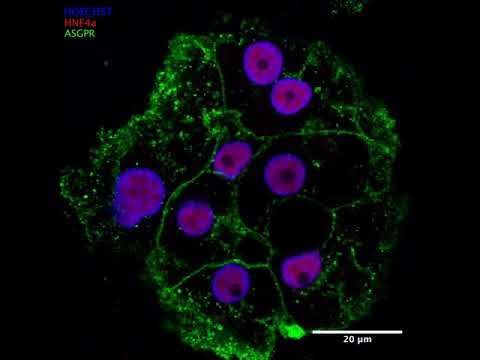
iPSC-derived hepatocytes are liver cells created from induced pluripotent stem cells (iPSCs). iPSCs are special because they can be generated from adult cells—like skin or blood cells—through a process called reprogramming. This involves introducing certain genes that revert these adult cells back to a pluripotent state, meaning they can turn into almost any cell type in the body. Once iPSCs are established, scientists can guide them to become hepatocytes, the main cells that make the liver function. These iPSC-derived hepatocytes are incredibly useful for a range of purposes: Disease Modeling: Researchers can create iPSCs from a patient’s own cells and turn them into hepatocytes to study liver diseases on a cellular level. Drug Testing and Toxicity Screening: These liver cells offer a human-based model to test how new drugs work and whether they’re safe, potentially reducing the need for animal testing. Regenerative Medicine: iPSC-derived hepatocytes could be key to developing cell-based therapies for liver diseases or even creating bioengineered liver tissue. Basic Research: By studying these cells, scientists can gain deeper insights into liver development, function, and the underlying mechanisms of liver diseases. In short, iPSC-derived hepatocytes are a powerful tool in biomedical research and therapeutic development, offering a personalized, human-relevant way to explore liver function and disease. Related MTMLab Publication: https://pubmed.ncbi.nlm.nih.gov/39082962/